
There are also reports of VNS being successfully utilized in patients with refractory and super-refractory status epilepticus. VNS may provide benefit for particular epilepsy syndromes and seizure types such as Lennox-Gastaut syndrome, tuberous sclerosis complex related epilepsy, refractory absence seizures and atonic seizures. Patients with comorbid depression have been found to have mood improvements with VNS therapy. Patients who have poor adherence or tolerance of anti-seizure medications may be good candidates for VNS. This is because epilepsy surgery is associated with a higher probability of resulting in seizure freedom.
#VAGAL NERVE STIMULATOR TRIAL#
It is recommended that VNS is only pursued following an adequate trial of at least 2 appropriately chosen anti-seizure medications and that the patient is ineligible for epilepsy surgery. In the European Union, VNS is approved as an adjunctive therapy for patients with either generalized or focal onset seizures without any age restrictions. In the United States, VNS is approved as adjunctive therapy for those 4 years of age or older with refractory focal onset seizures. VNS devices are used to treat drug-resistant epilepsy and treatment-resistant major depressive disorder (TR-MDD). It is used as an add-on treatment for certain types of intractable epilepsy and treatment-resistant depression.

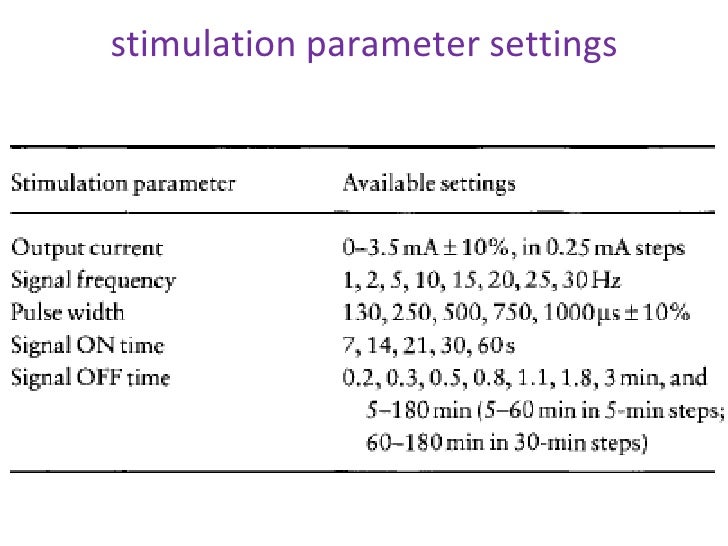
Research has shown that these side effects may occur in up to 29% of people in the first year to no more than 3% in the third year of VNS use. These usually occur when a person is getting used to the stimulation. Side effects of VNS are usually mild, including hoarseness and coughing, tingling, and shortness of breath. However, if the VNS works, some people can lower the number or dose of medications and lessen side effects. People continue to take seizure medications while using VNS. VNS is not considered a substitute for seizure medications. And in some people, it doesn’t work at all. Usually seizure control improves over time, with up to about 45% of people having seizures decrease at least by half after 1 to 2 years of therapy.Ĭomplete seizure freedom by VNS happens in only small numbers of people. The stimulation doesn’t work right away, but after a few months of therapy, about 25 to 30% of people may see that seizures decrease by half or more. A magnet can also be used to trigger bursts of stimulation when a seizure happens. Settings can be adjusted by a nurse and doctor trained in the use of VNS Therapy.
#VAGAL NERVE STIMULATOR GENERATOR#
Preprogrammed stimulation can be delivered from the generator in the chest to the vagus nerve. An electrode attached to the generator is then connected to the vagus nerve in the left side of the neck.
#VAGAL NERVE STIMULATOR SKIN#
VNS Therapy consists of a device placed under the skin near the collarbone in the left side of the chest. Food and Drug Administration (FDA) approved vagus nerve stimulation as a therapeutic intervention for intractable focal or partial epilepsy in people 12 years of age and over. Researchers later found that electrical stimulation of the vagus nerve can decrease seizures in animals, and clinical trials were done in humans. In the 19th century, scientists realized that seizures may stop by activating the vagus nerve when pressure was applied to the carotid artery in the neck. Important functions of the vagus nerve include controlling breathing and speech, regulating heartbeats, and regulating stomach movements during digestion. The vagus nerve sends information from your neck, chest, and stomach to the brain, and back from the brain to these regions. VNS Therapy, or vagus nerve stimulation, is a way of controlling seizures in people who do not respond to medications and may not respond to surgery.


 0 kommentar(er)
0 kommentar(er)
